Nanoparticles have potential applications in a wide range of fields, including electronics, medicine and material research, because of their remarkable and exceptional attributes. Carbon nanocones are planar carbon networks with mostly hexagonal faces and a few non-hexagonal faces (mostly pentagons) in the core. Two types of nanocone configurations are possible: symmetric and asymmetric, depending on where the pentagons are positioned within the structure. In addition to being a good substitute for carbon nanotubes, carbon nanocones have made an identity for themselves in a number of fields, including biosensing, electrochemical sensing, biofuel cells, supercapacitors, gas storage devices, and biomedical applications. Their astonishing chemical and physical attributes have made them well-known and widely accepted in the fields of condensed matter physics, chemistry, material science, and nanotechnology. Mathematical and chemical breakthroughs were made possible by the concept of modeling a chemical structure as a chemical graph and quantitatively analyzing the related graph using molecular descriptors. Molecular descriptors are useful in many areas of chemistry, biology, computer science, and other sciences because they allow for the analysis of chemical structures without the need for experiments. In this work, the quotient graph approach is used to establish the distance based descriptors of symmetrically configured two-pentagonal and three-pentagonal carbon nanocones.
Conical-shaped carbon nanostructures known as carbon nanocones were initially studied by Harris et al. [1]. Iijima et al. only identified them as such in 1999, despite the fact that Harris et al. had made the initial observation [2]. Single-walled and multi-walled carbon nanocones are the two different kinds of nanocones. In 1997, heavy oil was pyrolyzed in a carbon electric arc to create multi-walled carbon nanocones. In 1999, graphite was laser-ablated to create clustered nanocones[2]. Arc discharge and laser ablation are the two primary methods for synthesising carbon nanocones; however, a third method, such as Joule heating, has also been effectively used [3,4]. Because of its high yield, lack of purifying requirements, and low cost of production, large-scale commercial synthesis of nanocones is a suitable application [4].
Nanocones are planar graphs with a majority of hexagonal faces, along
with some non-hexagonal faces in core part. The non-hexagonal faces are
typically pentagons, though they can also be triangles, squares, and
other shapes. Different varieties of nanocones are produced based on the
presence of different non-hexagonal faces in the core part of the
nanocone structure. One of the major type is called as single
In comparison to other carbon nanostructures, these one-dimensional carbon nanostructures have better porosity, outstanding conductivity, high yield, high chemical stability, high purity, low toxicities, and remarkable catalytic capabilities. These remarkable qualities make nanocones a viable substitute for carbon nanostructures. Thus, in a variety of applications, they offer a practical and beneficial substitute for carbon nanotubes and potentially graphene. Because of all these characteristics, it broadened its applicability to a number of domains, including biofuel cells, supercapacitors, gas storage devices, biochemical sensing, and electrochemical sensing [6-9]. A distinct collection of structural, mechanical, chemical, and electrical properties are defined by the nanocone’s geometry. Determining these qualities requires a substantial amount of work. This work, which models a compound’s structure as a chemical graph and then conducts quantitative analysis using molecular descriptors, has become quite efficient with the advent of chemical graph theory. With the aid of their corresponding molecular graph, this establishes a relationship between the physicochemical qualities and the structure of the chemical substances through some helpful graph invariants.
The idea of topological descriptors was first introduced by Wiener in 1947 [10]. Eventually many other topological descriptors were introduced following the pioneering works of Wiener [10] and Randić [11]. These include distance based descriptors, degree based descriptors, connectivity based descriptors, etc. Also the degree and distance was combined by Schultz [12] and Gutman [13] and they came up with degree-distance based descriptors. Each descriptor is related with distinct properties of a chemical structure and hence, these descriptors can be used to investigate the relationships between structure, properties and activity of chemical compounds. Pharmaceutical and chemical techniques are rapidly developing in the current technological development era, resulting in the rapid emergence of new medicines, nanomaterials, crystalline compounds, and so on. To investigate and analyse the physical, chemical or biological properties of these arising structures requires different chemical experiments and huge effort. Pharmaceutical and chemical researchers need to put in a great effort inorder to cover these vast area of research. The chemical structure’s molecular descriptor is a non-empirical numerical quantity that quantifies the structure and its diverging arrangement and these indices can be considered as a total function which draws the molecular structure to an actual number. The applications of certain distance based molecular descriptors are given in [10, 11, 14,17]. It has found its application in quantitative structure-property relationships and quantitative structure-activity relationships. An important application of QSPR/QSAR models is that the properties, activities, behavior, etc. of a newly developed or untested chemical compound can be inferred from the molecular structure of similar compounds whose properties, activities, characteristics, etc have already been evaluated.
This paper considers carbon nanocones with two and three pentagons in
their core. The pentagons are positioned in such a way that, the
nanocone has symmetric configuration. Hence the nanocones are
represented as
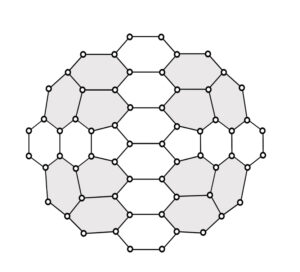
Consider a simple, finite, connected graph
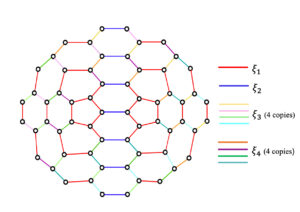
The concept of strength weighted graph is presented in [24]. A strength weighted graph is
denoted as
The values of
The distance based molecular descriptors and their corresponding
mathematical expression for strength-weighted graph
| Wiener | |
|---|---|
| Szeged | |
| Edge-Szeged | |
| Mostar | |
| Edge-Mostar | |
| Padmakar-Ivan |
This section comprises of theorems based on the distance based
molecular descriptors of symmetrically configured two pentagonal
nanocone,
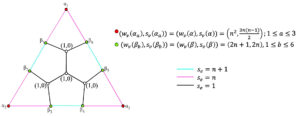
The symmetrically configured two pentagonal nanocone,

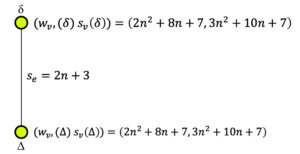

Theorem 1. For
Proof. The nanocone structure
For the quotient graph
Considering the second type of quotient graph
By using ([24]-Theorem 1),
the wiener index of
Theorem 2. For
Proof. As mentioned,
For quotient graph
For quotient graph
For quotient graph
By using ([24]-Theorem 1),
the szeged index of
Similarly using the expression for computing the edge-szeged index
from Table 1, the following can be determined:
Theorem 3. For
Proof.
For quotient graph
For quotient graph
For quotient graph
By using ([24]-Theorem 1),
the mostar index of
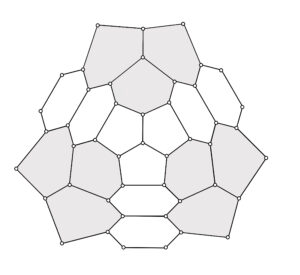
The symmetrically configured three pentagonal nanocone,
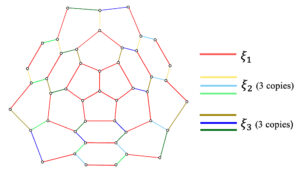
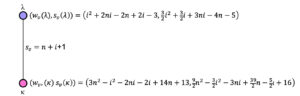
Theorem 4. For
Proof. The proof follows the similar pattern as in Theorem
1.
Theorem 5. For
Proof. The nanocone
Theorem 6. For
Proof. The quotient graphs of
Investigation a chemical structure experimentally is time consuming
as well as cost consuming. Determining molecular descriptors of the
chemical structure eases the procedure and is found to be an effective
way to analyse the structure. In this work, the distance based molecular
descriptors for symmetrically configured two pentagonal nanocone,
The authors did not receive support from any organization for the submitted work.
Authors declare no conflict of interest.
1970-2025 CP (Manitoba, Canada) unless otherwise stated.